Health
- Details
- Written by: American Lung Association
This January, during Radon Action Month, American Lung Association is raising awareness about radon and addressing common myths around this deadly gas.
“Hidden in far too many homes is the nation’s second-leading cause of lung cancer, radon. Lung cancer remains the nation’s leading cause of cancer deaths, so people need to be aware of and take action on radon,” said William Barrett, director of Clean Air Advocacy for American Lung Association in California. “The good news is that testing for and reducing the high radon levels is straightforward and effective. The Lung Association encourages all families, schools and daycares to test for radon to protect everyone’s health and save lives.”
Radon has been found in every county in California with several areas on the Central Coast registering potential elevated levels. Please help us bust these common myths about radon:
Myth No. 1: Radon is not really harmful.
Fact: Not only is radon invisible, it’s also radioactive. While you can’t see it, exposure to high levels of radon over time can cause lung cancer, and radon ranks as the nation’s second-leading cause of the disease. Radon-related lung cancers are responsible for an estimated 21,000 deaths every year in the United States.
Myth No. 2: Radon is rare and doesn’t impact our community.
Fact: The reality is that radon is found at dangerous levels in an estimated 1 in 15 homes nationwide. Your home can have elevated levels of radon while your neighbor’s home does not. It doesn’t matter in what part of the country you live, because radon comes from rock and soil, it can be found anywhere. It then enters the home or building through cracks in walls, basement floors, foundations and other openings, and can exist at dangerous levels indoors.
Myth No. 3: Testing for radon is expensive.
Fact: The only way to detect dangerous levels of radon in your home is to test the air. Various forms of do-it-yourself test kits are simple to use, inexpensive and can be purchased online or at home improvement and hardware stores. Professional testing is also available, often for under $300, although the price varies by location and building size. Schools and daycares may need professional help to do the testing.
Myth No. 4: Our schools are safe.
Fact: Testing for radon in schools is not required in most states, nor is fixing the problem. Not only children, but teachers and other staff who work in schools can be exposed to dangerous levels of radon. The last nationwide survey of radon levels in schools, completed in 1993, found that nearly one in five schools had at least one classroom with dangerous levels of radon. The American Lung Association leads a coalition of groups working to highlight the importance of testing for radon in both schools and daycares through the National Radon Action Plan. In 2014, federal actions have already reached an estimated 1.6 million homes, schools and childcare facilities with guidance and incentives to reduce radon risk and have tested for and mitigated high radon risk when necessary in nearly 200,000 units.
To learn more about radon and how to test homes, visit www.Lung.org/radon or call the toll-free Lung HelpLine at 1-800-LUNGUSA.
- Details
- Written by: California Attorney General’s Office
The settlement – of which California will receive $8 million – resolves allegations that the company violated state consumer protection laws by misrepresenting the effectiveness and safety of its hip implant devices.
The multistate settlement alleges that Johnson & Johnson conducted unfair and deceptive marketing practices by making misleading claims on the longevity – also known as survivorship – of its metal-on-metal hip implant devices. Johnson & Johnson has also agreed to injunctive terms to reform how it markets and promotes its hip implant products.
“Johnson & Johnson is alleged to have deceived vulnerable patients in need of hip replacement and undermined their ability to recuperate quickly and safely,” said Attorney General Becerra. “The company allegedly deceived consumers by circulating misleading research and ignoring up-to-date information about the effectiveness of its devices. There’s no excuse for Johnson & Johnson to have violated its customers’ trust, as well as California consumer protection laws, but we worked to hold them accountable.”
The multistate settlement resolves allegations that Johnson & Johnson violated state law by misleading consumers in the marketing of metal-on-metal hip implant devices used for hip replacement surgeries.
In 2005, Johnson & Johnson began marketing its ASR XL device to doctors seeking to provide longer-lasting hip replacement surgery in younger, more active patients. Johnson & Johnson actively and falsely advertised the product’s stability and survivorship as part of its “Never Stop Moving” campaign, citing an implant survivorship of nearly 100 percent after five years.
Johnson & Johnson also misrepresented the implant survivorship of another hip implant device, the Pinnacle Ultamet, relying upon a questionable 2007 study that was advertised as independent, but designed by Johnson & Johnson.
After receiving hip implants using these products, some consumers experienced painful side effects from the products, including persistent groin pain, allergy, tissue necrosis, as well as a build-up of metal ions in the blood.
As part of the settlement, Johnson & Johnson will pay $120 million in penalties, and comply with a set of important injunctive terms that are enforceable by the California Attorney General in the event of future misconduct. Under the consent judgement, the Johnson & Johnson subsidiary companies that market these devices will:
– Base claims of survivorship, stability or dislocations on scientific information and the most recent dataset available from a registry for any DePuy hip implant device;
– Maintain a post-market surveillance program and complaint handling program;
– Update and maintain internal product complaint handling operating procedures, including training of complaint reviewers;
– Update and maintain processes and procedures to track and analyze product complaints that do not meet the definition of Medical Device Reportable Events;
– Maintain a quality assurance program that includes an audit procedure for tracking complaints regarding DePuy Products that do not rise to the level of a Medical Device Reportable Event but that may indicate a device-related serious injury or malfunction; and
– Perform quarterly reviews of complaints, and if a subgroup of patients is identified that has a higher incidence of adverse events than the full patient population, determine the cause and alter promotional practices as appropriate.
This is the second settlement Attorney General Becerra has reached with Johnson & Johnson.
In May 2017, Attorney General Becerra announced a $33 million settlement with the company after it failed to ensure the quality of over-the-counter medications including Tylenol, Motrin, Benadryl, St. Joseph Aspirin, Sudafed, Pepcid, Mylanta, Rolaids, and Zyrtec.
The settlement is subject to court approval. A copy of the complaint can be found here.
- Details
- Written by: Robert Sanders
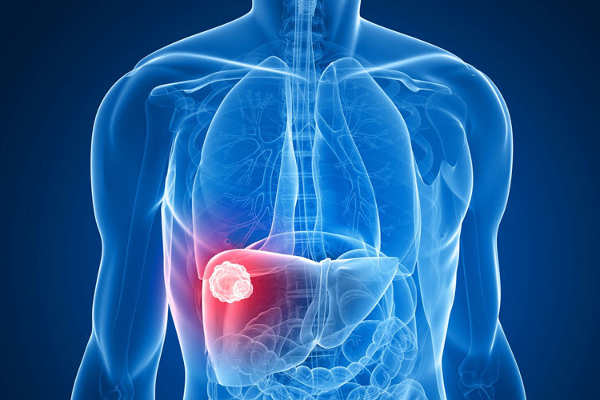
BERKELEY, Calif. – With the help of sponges inserted in the bloodstream to absorb excess drugs, doctors are hoping to prevent the dangerous side effects of toxic chemotherapy agents or even deliver higher doses to knock back tumors, like liver cancer, that don’t respond to more benign treatments.
The “drug sponge” is an absorbent polymer coating a cylinder that is 3D printed to fit precisely in a vein that carries the blood flowing out of the target organ – the liver in liver cancer, for example. There, it would sop up any drug not absorbed by the tumor, preventing it from reaching and potentially poisoning other organs.
In early tests in pigs, the polymer-coated drug absorber took up, on average, 64 percent of a liver cancer drug – the chemotherapy agent doxorubicin – injected upstream.
“Surgeons snake a wire into the bloodstream and place the sponge like a stent, and just leave it in for the amount of time you give chemotherapy, perhaps a few hours,” said Nitash Balsara, a professor of chemical and biomolecular engineering at the University of California, Berkeley, and a faculty scientist at Lawrence Berkeley National Laboratory.
“Because it is a temporary device, there is a lower bar in terms of approval by the FDA,” said Steven Hetts, an interventional radiologist at UC San Francisco who first approached Balsara in search of a way to remove drugs from the bloodstream. “I think this type of chemofilter is one of the shortest pathways to patients.”
Most anticancer drugs are poisonous, so doctors walk a delicate line when administering chemotherapy. A dose must be sufficient to kill or stop the growth of cancer cells, but not high enough to irreparably damage the patient’s other organs. Even so, chemotherapy is typically accompanied by major side effects, including nausea, vomiting, diarrhea and suppression of the immune system, not to mention hair loss and ulcers.
“We are developing this around liver cancer because it is a big public health threat – there are tens of thousands of new cases every year – and we already treat liver cancer using intra-arterial chemotherapy,” Hetts said. “But if you think about it, you could use this sort of approach for any tumor or any disease that is confined to an organ, and you want to absorb the drug on the venous side before it can distribute and cause side effects elsewhere in the body. Ultimately we would like to use this technology in other organs to treat kidney tumors and brain tumors.”
Hetts, Balsara and their colleagues at UC Berkeley, UCSF and the University of North Carolina, Chapel Hill, will publish their results today in the journal ACS Central Science, an open-access publication of the American Chemical Society.
Gentler treatments
Hetts, the chief of interventional neuroradiology at the UCSF Mission Bay Hospitals, treats tumors of the eye and brain by threading catheters through the bloodstream to deliver chemotherapy drugs directly to the site of the tumor. This delivers the maximum dose to the tumor and the least dose to the rest of the body, minimizing side effects. It is a vast improvement over injecting chemotherapy drugs straight into the bloodstream, which allows the drugs to reach and poison every part of the body and gambles on the tumor succumbing before the patient. Nevertheless, typically more than half of the dose injected into the body escapes the target organ.
Several years ago, he started thinking about a major improvement: filtering the blood coming out of the targeted organ to remove excess chemo so that much less of the drug reaches the body as a whole.
Balsara, a chemical engineer who specializes in ionic polymers for batteries and fuel cells, is one of the people Hetts approached to find a suitable absorber to put in the bloodstream. In 2016, former UC Berkeley and Berkeley Lab postdoctoral fellow Chelea Chen identified an ionic polymer, not unlike polymers used in fuel cells, that efficiently absorbed doxorubicin,
“An absorber is a standard chemical engineering concept,” Balsara said. “Absorbers are used in petroleum refining to remove unwanted chemicals such as sulfur. Literally, we’ve taken the concept out of petroleum refining and applied it to chemotherapy.”
That polymer led Balsara’s team to a commercial version of the absorbent polymer that was easier to obtain in large quantities, and Berkeley postdoc Hee Jeung Oh spent more than a year perfecting a way to adhere the polymer to a 3D-printed cylinder with crisscrossing struts that could be placed inside a person’s vein.
“Fitting the cylinder in the vein is important; if the fit is poor, then the blood with the dissolved drug will flow past the cylinder without interacting with the absorbent,” Balsara said. Recognizing the need for customizing the device for individual patients, Balsara solicited the help of a long-time collaborator, Joseph DeSimone, the CEO of Carbon, Inc., a 3D-printing company in Redwood City.
In the experiments reported in ACS Central Science, Hetts implanted the 3D-printed device into the vein of a pig and measured how much of the doxorubicin injected upstream remained downstream of the absorber. In a healthy pig, about 64 percent of the drug was removed.
They are currently in the midst of experiments to determine how much drug is absorbed when the device is implemented at the exit of a healthy pig liver, though the true test will be in humans, perhaps in a couple of years, Hetts said.
“This is a first level in vivo validation that yes, this device will bind up drug in the bloodstream,” he said. “But extensive animal testing is not the next path; the next path is getting conditional approval from FDA to do first-in-human studies, because it is much more realistic to test these in people who have cancer as opposed to continuing to test in young pigs who have otherwise healthy livers.”
Hetts says that the technique is superior to another liver cancer treatment now undergoing testing, which requires major endovascular surgery to completely block the outputs from the liver with balloons and divert the outflowing blood to an external dialysis machine, where the drug is removed and the blood returned to the body.
“There is a lot of opportunity to develop less-invasive devices that will bind up the drug in a gentler manner,” he said.
Drug sponges could be applied to many types of tumors and chemotherapy drugs, Hetts said, and could potentially be used to sop up other dangerous drugs, such as high-powered antibiotics that are toxic to the kidneys but required to kill a pathogen.
“We think this is a generally applicable concept,” he said.
The work was supported by a grant from the National Institutes of Health. Other coauthors are Mariam Aboian, Mark Wilson, Terilyn Moore and Colin Yee of UCSF; Michael Yi, Jacqueline Maslyn and Whitney Loo of UC Berkeley; Xi Jiang and Dilworth Parkinson of Berkeley Lab; and DeSimone, Gregory Robbins and Florian Barth of Carbon.
Robert Sanders writes for the UC Berkeley News Center.
- Details
- Written by: COVERED CALIFORNIA
“Covered California is in the final two days of open enrollment. That means if you are without health insurance, you need to sign up by Tuesday, Jan. 15, to secure health coverage,” Newsom said.
Newsom’s statement came one week after he unveiled a new health care agenda aimed at enhancing affordability in the years ahead and building on the Patient Protection and Affordable Care Act as a pathway to universal coverage.
Covered California Executive Director Peter V. Lee, who was in Los Angeles Monday to promote open enrollment, said time is of the essence.
“The clock is ticking for consumers who need quality health care coverage because this year’s deadline is earlier than it has been in the past,” Lee said. “You must take action today or tomorrow to start an application for health coverage.”
Lee praised the governor’s proposals to enhance affordability by increasing subsidies in the future for middle-income Californians.
“The governor is to be applauded for his agenda to make health insurance more affordable in the years ahead,” Lee said. “The changes he seeks would make a big difference in the support provided to existing Covered California enrollees as well as a new group of individuals who would get financial help to buy health insurance for the first time.”
Gov. Newsom urged Californians to visit www.CoveredCA.com for more information.
“Covered California is the only place you can go to see if you qualify for financial help that can take hundreds of dollars off the price of your insurance premium,” he said. “An estimated 1.1 million Californians are eligible for quality health care coverage, either through Covered California or Medi-Cal, so do not miss this chance to get coverage that will protect you and your family,” he said.
Open enrollment is the one time of the year when consumers can sign up for coverage without needing to meet any conditions. California’s previous open-enrollment period ran through January, but a state law established that open enrollment would run from Oct. 15 through Jan. 15 each year.
Covered California made a stop in Los Angeles on Monday to promote enrollment with longtime partner Asian Americans Advancing Justice. The event culminated a bus tour that traveled more than 2,000 miles, with stops in cities across the state.
The tour will feature individuals who have been enrolled through Covered California since the exchange first opened its doors. Local dance crews will also be depicting the idea that life can change in an instant, which ties to Covered California’s award-winning advertising campaign that features ads showing individuals slipping down stairs, falling off a ladder, getting in a bicycle accident and cutting their hand in the kitchen.
Watch and download time-lapse video of the Covered California bus being wrapped.
“Dance transcends culture, language and age,” Lee said. “These performances from around the state will help Covered California encourage enrollment using a medium that resonates with Californians.”
There will be two performances in Los Angeles, at the headquarters of Asian Americans Advancing Justice. Both dances will be performed by the world-famous GRV dance crew. The crew and the choreographers, Eileen Kim and David Lim, are all based in Los Angeles. Both Kim and Lim have spent years dancing, teaching and touring the world with their performances.
In addition to the live performances during the bus tour, the dances will be captured on video and shared through social media and on Covered California’s website.
“Californians will be able to share these dramatic performances with their family and friends,” Lee said. “We hope the videos spark conversations and get people interested in seeing how affordable it can be to get quality coverage.”
- Senate leader introduces bill to ease access to mental health care through Medi-Cal
- California’s open enrollment for individuals ends Jan. 15; consumers have one week to sign up for health care coverage
- Department of Insurance issues report on the impact of prescription drug costs on health insurance premiums