Health
The U.S. Department of Energy announced that its support for a decade of revolutionary research has contributed to the creation of the first ever retinal prosthesis – or bionic eye – to be approved in the United States by the U.S. Food and Drug Administration for blind individuals with end-stage retinitis pigmentosa.
“The development of the artificial retina is just one more example of the unique value of our National Laboratories and research universities,” said Energy Secretary Steven Chu. “While no one can predict a breakthrough before it happens, the investments we’re making in research pay enormous dividends for our economy and improve our lives.”
The artificial retina, dubbed the Argus II Retinal Prosthesis System (developed and manufactured by Second Sight Medical Products Inc, Sylmar, California) may prove to be an aid to those blinded by the disease retinitis pigmentosa, which can run in families and is estimated by the National Institutes of Health to affect about 1 in 4,000 people in the United States.
Over the 10 year lifetime of the project, the Department provided $75.2 million for the development of technologies aimed at advancing artificial retinas like the Argus II, which was based on work by a consortium of scientists using advanced technologies developed by several of the Department’s National Laboratories.
The Argus II can partially restore the sight of blind individuals after surgical implantation. Clinical trials demonstrated that totally blind individuals could safely use the device to successfully identify the position and approximate size of objects and detect movement of nearby objects and people.
The Argus II operates by using a miniature camera mounted in eyeglasses that captures images and wirelessly sends the information to a microprocessor (worn on a belt) that converts the data to an electronic signal and transmits it to a receiver on the eye.
The pulses travel to the optic nerve and, ultimately, to the brain, which perceives patterns of light and dark spots corresponding to the electrodes stimulated. Blind individuals can learn to interpret these visual patterns.
The $75.2 million in funding for the 10-year project was provided through DOE’s Office of Science.
The project, conducted under a Cooperative Research and Development Agreement with the private sector company Second Sight, included researchers from five DOE National Laboratories – Argonne National Laboratory in Argonne, Ill.; Lawrence Livermore National Laboratory in Livermore, Calif.; Los Alamos National Laboratory in Los Alamos, New Mexico; Oak Ridge National Laboratory in Oak Ridge, Tenn.; and Sandia National Laboratories in Albuquerque, New Mexico and five universities – California Institute of Technology in Pasadena; Doheny Eye Institute at the University of Southern California in Los Angeles; North Carolina State University in Raleigh, North Carolina; University of Utah in Salt Lake City; and the University of California in Santa Cruz.
DOE also worked closely with the National Eye Institute at the National Institutes of Health to support the development of the Argus II and the National Science Foundation provided support for material design and other basic research.
- Details
- Written by: John Jensen
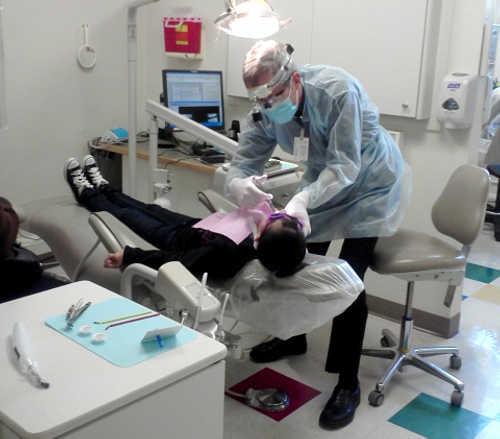
UKIAH, Calif. – On Saturday, Feb. 2, Mendocino Community Health Clinic (MCHC) continued its tradition of caring for the most vulnerable people in our community by participating in the annual Give Kids a Smile Day.
On the first Saturday of February, dental staff from all three sites – in Ukiah, Willits and Lakeport – volunteered their time to provide thousands of dollars worth of free dental care to children who could not otherwise afford it.
“We always look forward to Give Kids a Smile Day,” said Dental Manager Becky Driscoll. “It’s a lot of work, but it’s totally worth it. This year we provided more than $15,000 of free dental care to 33 pediatric patients.”
The event requires preparation in identifying qualified patients, setting up appointments, preparing equipment and supplies, and cleaning and sterilizing everything after the patients leave.
Dental Director Dr. Doug Lewis thanked the dentists and then made a special point to recognize the employees who support the event, saying, “The real heroes are the staff: the patient service representatives, dental assistants, sterile techs, and HealthCorps navigators. They organize it, they arrange the patients, they set up the rooms, and then they shut everything down at the end.”
Give Kids a Smile Day is a national event in which thousands of dentists and their teams provide free oral health care services to children from low-income families.
In February and throughout the year, MCHC provides dental care to children.
For more information, call 707-468-1010.
- Details
- Written by: Editor
SACRAMENTO – Assemblymember Mariko Yamada (D–Davis), chair of the Assembly Committee on Aging & Long-Term Care, has introduced the Home Care Services Act of 2013.
AB 322, co-authored by Senator Lou Correa (D–Santa Ana), establishes regulations for private home care agencies.
“California is aging, and is already home to the largest population of dependent adults in the nation,” Yamada said. “There should be clear standards for training and background checks for agency-based caregivers who wish to serve this vulnerable population.”
AB 322 authorizes the California Department of Social Services to license and regulate private home care agencies that hire and match home care aides with clients that need nonmedical assistance to live at home independently.
Many seniors and persons with disabilities, or their families, choose private home care agencies to secure the services of a comprehensively screened professional caregiver and avoid the liabilities and tax consequences of being an employer of an independent caregiver.
Currently, the only requirement to establish a homecare agency in California is a business license.
The lack of oversight and regulation of private homecare agencies can leave consumers without safeguards to protect them from negligent or criminal conduct.
The Home Care Services Act of 2013 responds to an industry call for regulation.
Assemblymember Yamada represents the Fourth Assembly District which includes all or parts of Colusa, Lake, Napa, Solano, Sonoma and Yolo counties.
- Details
- Written by: Editor
The Muscular Dystrophy Association has awarded a $400,000 infrastructure grant to National Institutes of Health (NIH) researchers to support exome sequencing on samples taken from 1,000 people with amyotrophic lateral sclerosis (ALS).
Data generated by the project will be made publicly available online and is expected to accelerate the pace of ALS research by helping scientists identify genes associated with the disease.
The MDA funding will enhance research conducted by the National Institute on Aging (NIA, part of the NIH) to better understand the genetic basis of neuromuscular disorders. The project will be led by Bryan Traynor, Ph.D., of the NIA Intramural Research Program in Bethesda, Md.
“It’s exciting to fund such an important project that will significantly expand the existing resources available for scientists to use in their ongoing efforts to better understand ALS,” said MDA Vice President of Research Jane Larkindale. “MDA is pleased to be able to collaborate with the NIH on this unique, first-of-its-kind endeavor.”
Exome-sequencing technology decodes stretches of DNA called exons, which contain instructions used in protein synthesis. Although exons make up only about 1.5 percent of the genome, the vast majority of disease-causing mutations occur in these sections. Exome data can be used to identify genes associated with human diseases.
“The whole team is tremendously excited about this project, which will establish a unique resource for the ALS research community aimed at accelerating gene discovery for this devastating disease,” said Traynor, who heads the Neuromuscular Disease Research Unit in the NIA’s Laboratory of Neuroscience. “Understanding the genetics underlying ALS is essential to understanding its onset and progression and in ultimately developing therapies to help patients.”
The exome-sequencing project is expected to be completed within 12 months. In that time, it will produce exome data from post-mortem tissue samples for 360 people with the sporadic form of ALS who have donated tissues to the NIH and for 640 additional tissue samples from sporadic ALS patients who have donated tissues to the Coriell ALS Repository.
Exome-sequencing data from a large number of people unaffected by ALS will be used for comparison in analysis.
MDA’s $400,000 infrastructure grant to support the project was made through the Association’s translational research program. To learn more, read the MDA/ALS Newsmagazine article “Unique ALS ‘Exome-Sequencing’ Project Is Focus of New Grant.”
Amyotrophic lateral sclerosis (also known as Lou Gehrig’s disease) attacks the nerve cells that control muscles, ultimately resulting in paralysis of all voluntary muscles, including those used for breathing.
Only about 5 percent of ALS is familial (where there is a history of ALS in more than one family member) with the other 95 percent occurring sporadically (without any family history of the disease). Average life expectancy for people with the disease is three to five years after diagnosis.
- Details
- Written by: Editor