Health
- Details
- Written by: Kara Manke
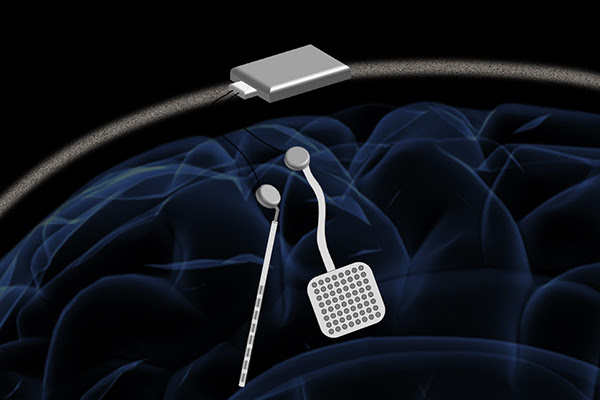
BERKELEY, Calif. – A new neurostimulator developed by engineers at the University of California, Berkeley, can listen to and stimulate electric current in the brain at the same time, potentially delivering fine-tuned treatments to patients with diseases like epilepsy and Parkinson’s.
The device, named the WAND, works like a “pacemaker for the brain,” monitoring the brain’s electrical activity and delivering electrical stimulation if it detects something amiss.
These devices can be extremely effective at preventing debilitating tremors or seizures in patients with a variety of neurological conditions. But the electrical signatures that precede a seizure or tremor can be extremely subtle, and the frequency and strength of electrical stimulation required to prevent them is equally touchy. It can take years of small adjustments by doctors before the devices provide optimal treatment.
WAND, which stands for wireless artifact-free neuromodulation device, is both wireless and autonomous, meaning that once it learns to recognize the signs of tremor or seizure, it can adjust the stimulation parameters on its own to prevent the unwanted movements. And because it is closed-loop — meaning it can stimulate and record simultaneously — it can adjust these parameters in real-time.
“The process of finding the right therapy for a patient is extremely costly and can take years. Significant reduction in both cost and duration can potentially lead to greatly improved outcomes and accessibility,” said Rikky Muller assistant professor of electrical engineering and computer sciences at Berkeley. “We want to enable the device to figure out what is the best way to stimulate for a given patient to give the best outcomes. And you can only do that by listening and recording the neural signatures.”
WAND can record electrical activity over 128 channels, or from 128 points in the brain, compared to eight channels in other closed-loop systems. To demonstrate the device, the team used WAND to recognize and delay specific arm movements in rhesus macaques. The device is described in a study that appeared December 31 in Nature Biomedical Engineering.
Ripples in a pond
Simultaneously stimulating and recording electrical signals in the brain is much like trying to see small ripples in a pond while also splashing your feet — the electrical signals from the brain are overwhelmed by the large pulses of electricity delivered by the stimulation.
Currently, deep brain stimulators either stop recording while delivering the electrical stimulation, or record at a different part of the brain from where the stimulation is applied — essentially measuring the small ripples at a different point in the pond from the splashing.
“In order to deliver closed-loop stimulation-based therapies, which is a big goal for people treating Parkinson’s and epilepsy and a variety of neurological disorders, it is very important to both perform neural recordings and stimulation simultaneously, which currently no single commercial device does,” said former UC Berkeley postdoctoral associate Samantha Santacruz, who is now an assistant professor at the University of Texas in Austin.
Researchers at Cortera Neurotechnologies, Inc., led by Rikky Muller, designed the WAND custom integrated circuits that can record the full signal from both the subtle brain waves and the strong electrical pulses. This chip design allows WAND to subtract the signal from the electrical pulses, resulting in a clean signal from the brain waves.
Existing devices are tuned to record signals only from the smaller brain waves and are overwhelmed by the large stimulation pulses, making this type of signal reconstruction impossible.
“Because we can actually stimulate and record in the same brain region, we know exactly what is happening when we are providing a therapy,” Muller said.
In collaboration with the lab of electrical engineering and computer science professor Jan Rabaey, the team built a platform device with wireless and closed-loop computational capabilities that can be programmed for use in a variety of research and clinical applications.
In experiments lead by Santacruz while a postdoc at UC Berkeley, and by and electrical engineering and computer science professor Jose Carmena, subjects were taught to use a joystick to move a cursor to a specific location. After a training period, the WAND device was capable of detecting the neural signatures that arose as the subjects prepared to perform the motion, and then deliver electrical stimulation that delayed the motion.
“While delaying reaction time is something that has been demonstrated before, this is, to our knowledge, the first time that it has been demonstrated in a closed-loop system based on a neurological recording only,” Muller said.
“In the future we aim to incorporate learning into our closed-loop platform to build intelligent devices that can figure out how to best treat you, and remove the doctor from having to constantly intervene in this process,” said Muller said.
Andy Zhou and Benjamin C. Johnson of UC Berkeley join Santacruz as co-lead authors on the paper. Other contributing authors include George Alexandrov, Ali Moin and Fred L. Burghardt of UC Berkeley. This work was supported in part by the Defense Advanced Research Projects Agency (W911NF-14- 2- 0043) and the National Science Foundation Graduate Research Fellowship Program (Grant No. 1106400). Authors Benjamin C. Johnson, Jan M. Rabaey, Jose M. Carmena and Rikky Muller have financial interest in Cortera Neurotechnologies, Inc., which has filed a patent application on the integrated circuit used in this work.
- Details
- Written by: Hillside Health Center
UKIAH, Calif. – With the addition of nurse practitioner Lily Robison, Hillside Health Center has once again expanded access to family medicine in Ukiah.
Robison, originally from the Democratic Republic of Congo, left the war-torn nation at age 16 and arrived in Salt Lake City, Utah. She spoke no English but hoped to follow her passion for medicine, nonetheless.
As a little girl, she set her sights on becoming a doctor, but when her life was upended with a move to the United States, she began adjusting her plans. After high school, she worked as a pharmacy technician while earning a bachelor’s degree in biology from the University of Utah.
During that time, the pharmacist she worked for recommended Robison pursue nursing school, which would allow her to fulfill her interest in caring directly for patients.
She achieved her bachelor’s degree in nursing in 2012, graduating as a member of the nursing honor society Sigma Theta Tau.
During nursing school, she enjoyed the patient interaction, but she yearned for a deeper, longer-term relationship with patients, and she could see the physical toll bedside nursing took, so she pursued a master’s degree in nursing and became a family medicine nurse practitioner.
Being a family nurse practitioner allows Robison to meet her personal and professional goals.
On the personal side, as the mother of two small children, Robison enjoys the regular schedule that comes with being a nurse practitioner because it allows her to spend time at home.
On the professional side, being a nurse practitioner allows her to work in a field she has always wanted to work in and to build the relationships she finds rewarding.
“It’s so satisfying seeing patients,” she said. “Regardless of our backgrounds, we all have problems, family challenges. To be in medicine, you must have empathy and you have to really listen to your patients.”
Robison decided to move across the country to work at MCHC Health Centers because she liked the people there, and the practice style felt like a good fit. She also liked the area. Her husband is from a small town in Wyoming and they wanted to raise their children in a similar environment.
Robison describes herself as family-oriented, conservative, and hard-working. “I’m quiet until you get to know me; then I talk a mile a minute,” she admitted.
Hillside Health Center is part of MCHC Health Centers – a local, nonprofit, federally qualified health center offering medical, dental and behavioral health care to people in Lake and Mendocino counties.
- Details
- Written by: American Lung Association
This January, during Radon Action Month, American Lung Association is raising awareness about radon and addressing common myths around this deadly gas.
“Hidden in far too many homes is the nation’s second-leading cause of lung cancer, radon. Lung cancer remains the nation’s leading cause of cancer deaths, so people need to be aware of and take action on radon,” said William Barrett, director of Clean Air Advocacy for American Lung Association in California. “The good news is that testing for and reducing the high radon levels is straightforward and effective. The Lung Association encourages all families, schools and daycares to test for radon to protect everyone’s health and save lives.”
Radon has been found in every county in California with several areas on the Central Coast registering potential elevated levels. Please help us bust these common myths about radon:
Myth No. 1: Radon is not really harmful.
Fact: Not only is radon invisible, it’s also radioactive. While you can’t see it, exposure to high levels of radon over time can cause lung cancer, and radon ranks as the nation’s second-leading cause of the disease. Radon-related lung cancers are responsible for an estimated 21,000 deaths every year in the United States.
Myth No. 2: Radon is rare and doesn’t impact our community.
Fact: The reality is that radon is found at dangerous levels in an estimated 1 in 15 homes nationwide. Your home can have elevated levels of radon while your neighbor’s home does not. It doesn’t matter in what part of the country you live, because radon comes from rock and soil, it can be found anywhere. It then enters the home or building through cracks in walls, basement floors, foundations and other openings, and can exist at dangerous levels indoors.
Myth No. 3: Testing for radon is expensive.
Fact: The only way to detect dangerous levels of radon in your home is to test the air. Various forms of do-it-yourself test kits are simple to use, inexpensive and can be purchased online or at home improvement and hardware stores. Professional testing is also available, often for under $300, although the price varies by location and building size. Schools and daycares may need professional help to do the testing.
Myth No. 4: Our schools are safe.
Fact: Testing for radon in schools is not required in most states, nor is fixing the problem. Not only children, but teachers and other staff who work in schools can be exposed to dangerous levels of radon. The last nationwide survey of radon levels in schools, completed in 1993, found that nearly one in five schools had at least one classroom with dangerous levels of radon. The American Lung Association leads a coalition of groups working to highlight the importance of testing for radon in both schools and daycares through the National Radon Action Plan. In 2014, federal actions have already reached an estimated 1.6 million homes, schools and childcare facilities with guidance and incentives to reduce radon risk and have tested for and mitigated high radon risk when necessary in nearly 200,000 units.
To learn more about radon and how to test homes, visit www.Lung.org/radon or call the toll-free Lung HelpLine at 1-800-LUNGUSA.
- Details
- Written by: California Attorney General’s Office
The settlement – of which California will receive $8 million – resolves allegations that the company violated state consumer protection laws by misrepresenting the effectiveness and safety of its hip implant devices.
The multistate settlement alleges that Johnson & Johnson conducted unfair and deceptive marketing practices by making misleading claims on the longevity – also known as survivorship – of its metal-on-metal hip implant devices. Johnson & Johnson has also agreed to injunctive terms to reform how it markets and promotes its hip implant products.
“Johnson & Johnson is alleged to have deceived vulnerable patients in need of hip replacement and undermined their ability to recuperate quickly and safely,” said Attorney General Becerra. “The company allegedly deceived consumers by circulating misleading research and ignoring up-to-date information about the effectiveness of its devices. There’s no excuse for Johnson & Johnson to have violated its customers’ trust, as well as California consumer protection laws, but we worked to hold them accountable.”
The multistate settlement resolves allegations that Johnson & Johnson violated state law by misleading consumers in the marketing of metal-on-metal hip implant devices used for hip replacement surgeries.
In 2005, Johnson & Johnson began marketing its ASR XL device to doctors seeking to provide longer-lasting hip replacement surgery in younger, more active patients. Johnson & Johnson actively and falsely advertised the product’s stability and survivorship as part of its “Never Stop Moving” campaign, citing an implant survivorship of nearly 100 percent after five years.
Johnson & Johnson also misrepresented the implant survivorship of another hip implant device, the Pinnacle Ultamet, relying upon a questionable 2007 study that was advertised as independent, but designed by Johnson & Johnson.
After receiving hip implants using these products, some consumers experienced painful side effects from the products, including persistent groin pain, allergy, tissue necrosis, as well as a build-up of metal ions in the blood.
As part of the settlement, Johnson & Johnson will pay $120 million in penalties, and comply with a set of important injunctive terms that are enforceable by the California Attorney General in the event of future misconduct. Under the consent judgement, the Johnson & Johnson subsidiary companies that market these devices will:
– Base claims of survivorship, stability or dislocations on scientific information and the most recent dataset available from a registry for any DePuy hip implant device;
– Maintain a post-market surveillance program and complaint handling program;
– Update and maintain internal product complaint handling operating procedures, including training of complaint reviewers;
– Update and maintain processes and procedures to track and analyze product complaints that do not meet the definition of Medical Device Reportable Events;
– Maintain a quality assurance program that includes an audit procedure for tracking complaints regarding DePuy Products that do not rise to the level of a Medical Device Reportable Event but that may indicate a device-related serious injury or malfunction; and
– Perform quarterly reviews of complaints, and if a subgroup of patients is identified that has a higher incidence of adverse events than the full patient population, determine the cause and alter promotional practices as appropriate.
This is the second settlement Attorney General Becerra has reached with Johnson & Johnson.
In May 2017, Attorney General Becerra announced a $33 million settlement with the company after it failed to ensure the quality of over-the-counter medications including Tylenol, Motrin, Benadryl, St. Joseph Aspirin, Sudafed, Pepcid, Mylanta, Rolaids, and Zyrtec.
The settlement is subject to court approval. A copy of the complaint can be found here.






 How to resolve AdBlock issue?
How to resolve AdBlock issue?